Ahmedabad, Gujarat
| Business Type | Manufacturer, Exporter, Supplier, Retailer, Wholesaler |
| Type | Active Pharma Ingredient |
| Color | White to off white powder |
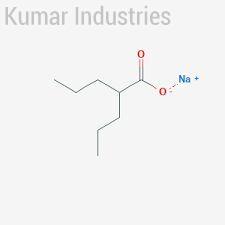
| CAS No. | 76584-70-8 |
| Click to view more | |
Preferred Buyer From
| Location | India, Afghanistan, Bahrain, Bangladesh, China, Hong Kong, India, Indonesia, Iran (Islamic Republic of), Iraq, Israel, Japan, Jordan, Kuwait, Malaysia, Nepal, Oman, Pakistan, Philippines, Saudi Arabia, Singapore, Korea, Republic of, Sri Lanka, Syrian Arab Republic, Taiwan, Thailand, Turkey, United Arab Emirates, Viet Nam, Yemen, France, Germany, Greece, Italy, Netherlands, Poland, Romania, Russian Federation, Spain, Ukraine, United Kingdom |
Product Details
Purity
>99%
Appearance
Powder / Granules
Standards
IP/USP
CAS. No
76584-70-8
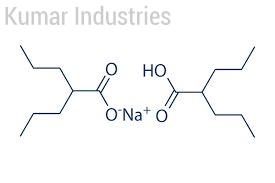
Molecular Formula
C16H31NaO4
Grade
Medicine Grade
Packaging Details
Divalproex sodium is a stable coordination compound comprised of sodium valproate and valproic acid used to treat manic episodes associated with bipolar disorder, epilepsy, and migraine headaches.
Looking for "Divalproex Sodium - IP/USP" ?
Explore More Products